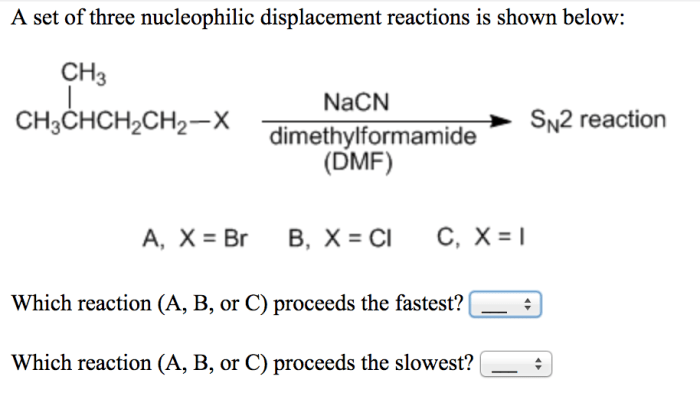
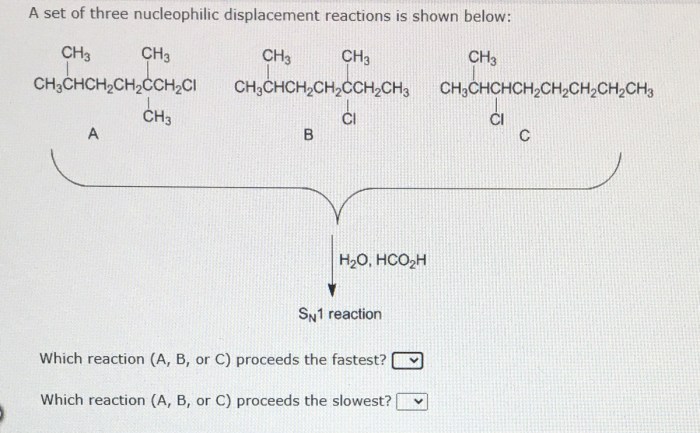
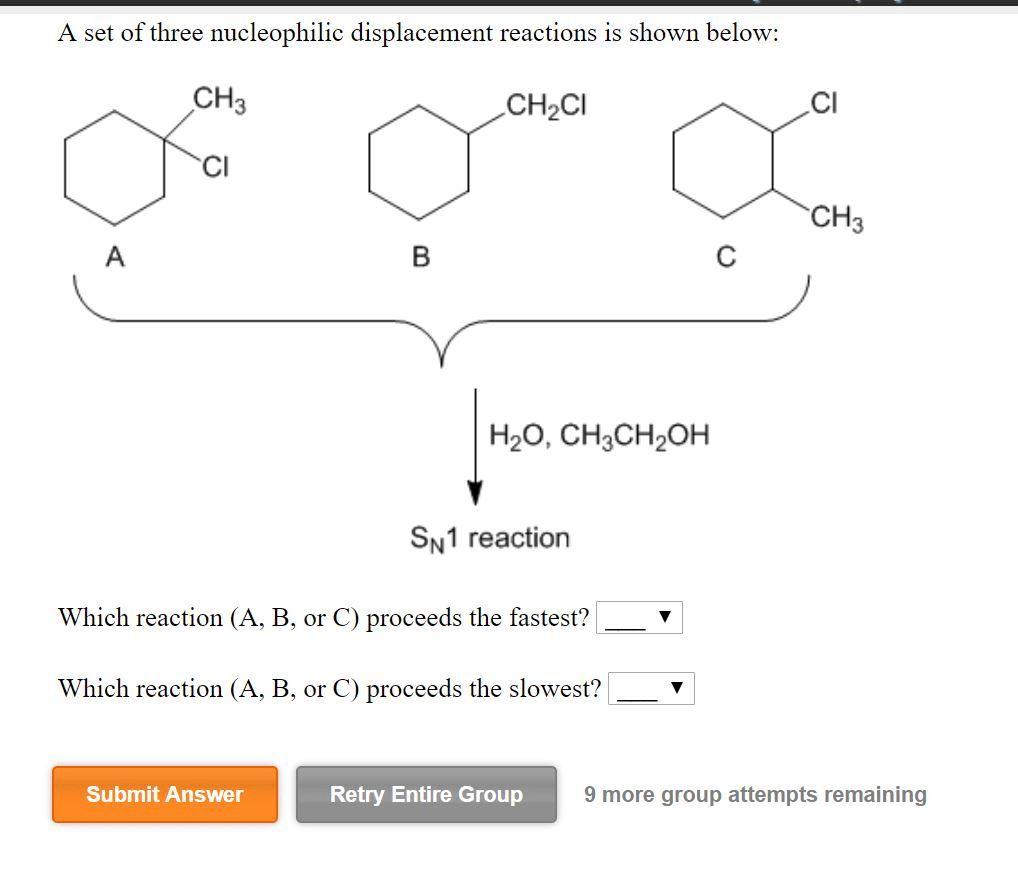
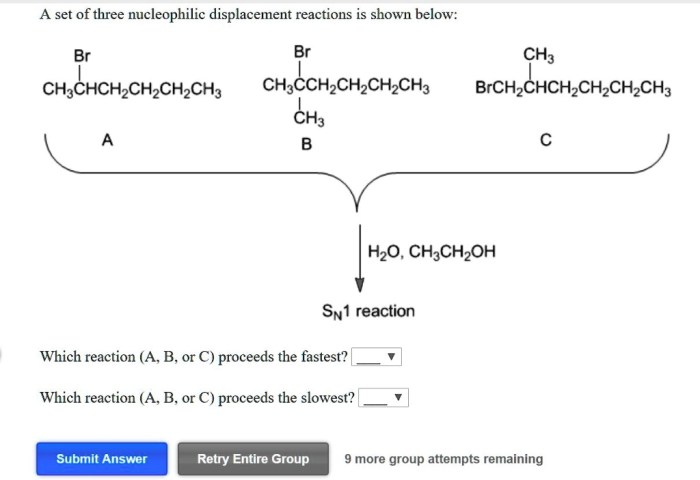
A set of three nucleophilic displacement reactions is shown below: this topic delves into the fascinating realm of nucleophilic displacement reactions, providing a comprehensive analysis of their mechanisms, factors influencing their reactivity, and their significance in various fields.
These reactions are ubiquitous in organic chemistry and play a crucial role in the synthesis of complex molecules. Understanding their intricacies is essential for comprehending the behavior of organic compounds and designing efficient synthetic strategies.
Overview of Nucleophilic Displacement Reactions
Nucleophilic displacement reactions are a fundamental class of reactions in organic chemistry. They involve the substitution of a leaving group by a nucleophile, resulting in the formation of a new bond between the nucleophile and the electrophile. The general mechanism of these reactions involves the nucleophile attacking the electrophile, leading to the formation of a transition state and ultimately the substitution product.
Common nucleophiles include hydroxide ions (OH-), alkoxide ions (RO-), and amines (RNH2), while electrophiles can be alkyl halides (R-X), acid chlorides (RCOCl), and epoxides (R-O-R). The nature of the nucleophile and electrophile, as well as the reaction conditions, influence the rate and selectivity of these reactions.
Analysis of the Given Reactions
The given set of three nucleophilic displacement reactions can be analyzed as follows:
- Reaction 1:
- Nucleophile: Hydroxide ion (OH-)
- Electrophile: Methyl bromide (CH3Br)
- Leaving group: Bromide ion (Br-)
- Product: Methanol (CH3OH)
- Reaction 2:
- Nucleophile: Sodium ethoxide (CH3CH2O-)
- Electrophile: Ethyl chloride (CH3CH2Cl)
- Leaving group: Chloride ion (Cl-)
- Product: Diethyl ether (CH3CH2OCH2CH3)
- Reaction 3:
- Nucleophile: Pyridine (C5H5N)
- Electrophile: Acetyl chloride (CH3COCl)
- Leaving group: Chloride ion (Cl-)
- Product: N-Acetylpyridine (CH3CONHC5H5)
Factors that influence the rate and selectivity of these reactions include the strength of the nucleophile, the reactivity of the electrophile, and the solvent effects.
Mechanistic Pathways
The step-by-step mechanism for each reaction involves the following steps:
- Reaction 1:
- Nucleophilic attack: The hydroxide ion attacks the methyl bromide, forming a transition state.
- Bond formation: The bond between the carbon and bromine atoms breaks, and a new bond forms between the carbon and oxygen atoms.
- Leaving group departure: The bromide ion leaves as a leaving group.
- Reaction 2:
- Nucleophilic attack: The sodium ethoxide attacks the ethyl chloride, forming a transition state.
- Bond formation: The bond between the carbon and chlorine atoms breaks, and a new bond forms between the carbon and oxygen atoms.
- Leaving group departure: The chloride ion leaves as a leaving group.
- Alkoxide attack: The alkoxide ion attacks the second carbon atom, forming a new bond and displacing the chloride ion.
- Reaction 3:
- Nucleophilic attack: The pyridine attacks the acetyl chloride, forming a transition state.
- Bond formation: The bond between the carbon and chlorine atoms breaks, and a new bond forms between the carbon and nitrogen atoms.
- Leaving group departure: The chloride ion leaves as a leaving group.
The structure of the substrate and nucleophile can affect the reaction pathway by influencing the steric hindrance and electronic effects.
Stereochemistry and Regioselectivity
The stereochemical outcome of nucleophilic displacement reactions is determined by the reaction mechanism. For example, in the reaction of an alkyl halide with a strong nucleophile, the product is typically a mixture of enantiomers due to the formation of a planar transition state.
However, in the case of an SN2 reaction, the product is typically a single enantiomer due to the inversion of configuration at the reaction center.
The regioselectivity of nucleophilic displacement reactions is influenced by the steric and electronic effects of the substrate. For example, in the reaction of a tertiary alkyl halide with a nucleophile, the reaction proceeds via an SN1 mechanism, and the product is a mixture of regioisomers due to the formation of a carbocation intermediate.
Applications and Importance
Nucleophilic displacement reactions are widely used in organic synthesis for the preparation of various compounds. For example, they are used in the synthesis of alcohols, ethers, esters, and amides. These reactions are also important in various fields, such as pharmaceuticals, materials science, and biotechnology.
Clarifying Questions: A Set Of Three Nucleophilic Displacement Reactions Is Shown Below:
What are nucleophilic displacement reactions?
Nucleophilic displacement reactions are a class of organic reactions in which a nucleophile (an electron-rich species) attacks an electrophile (an electron-poor species), resulting in the displacement of a leaving group and the formation of a new bond between the nucleophile and the electrophile.
What are the factors that influence the rate of nucleophilic displacement reactions?
The rate of nucleophilic displacement reactions is influenced by several factors, including the strength of the nucleophile, the reactivity of the electrophile, the nature of the leaving group, and the reaction conditions (temperature, solvent, etc.).
What are the applications of nucleophilic displacement reactions?
Nucleophilic displacement reactions are widely used in organic synthesis for the preparation of various compounds, including pharmaceuticals, dyes, and polymers. They are also employed in analytical chemistry for the identification and quantification of organic compounds.