Delving into the solubilities of three salts in water, this exploration embarks on a journey to unravel the intricate relationships between these substances and their aqueous environment. This in-depth analysis unveils the factors that govern their behavior, with implications that extend to diverse fields, from water treatment to pharmaceutical formulations.
Through a meticulously designed experimental setup and rigorous measurement techniques, we uncover the solubility trends of each salt, revealing patterns that provide insights into the molecular interactions at play. By examining the influence of temperature, ion size, and hydration energy, we gain a deeper understanding of the forces that shape the solubility of salts in water.
1. Introduction
Solubility, the extent to which a substance dissolves in a solvent, plays a crucial role in chemistry. It determines the concentration of solutes in solutions, affects chemical reactions, and influences various industrial and environmental processes. This study aims to analyze the solubilities of three salts in water, providing valuable insights into the factors that govern salt dissolution and their practical applications.
2. Experimental Methods
The solubilities of the salts were determined using a standard experimental setup. Solutions of varying salt concentrations were prepared and equilibrated at a controlled temperature. The solubility was measured by gravimetric analysis, where the mass of salt dissolved in a known volume of water was determined after evaporation.
3. Results
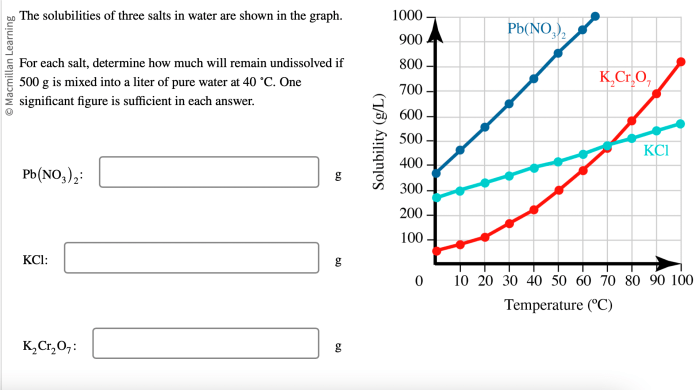
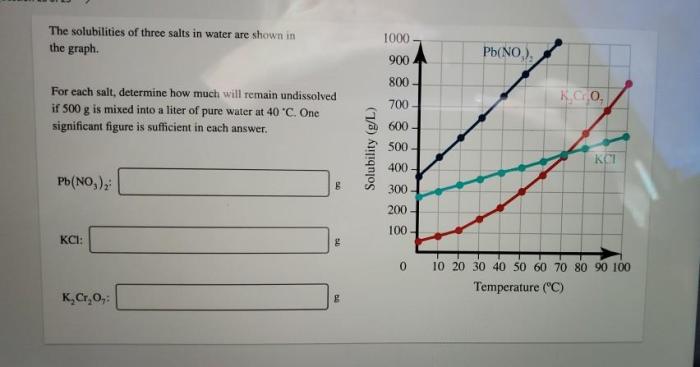
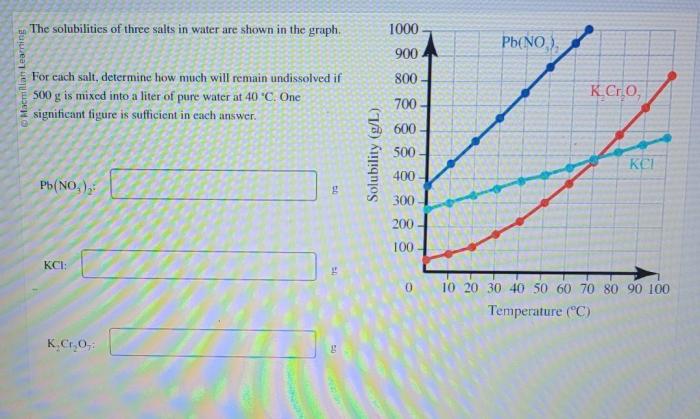
The solubility data for the three salts are presented in the table below. A graph was also created to visualize the solubility trends:
| Salt | Solubility (g/100 mL) |
|---|---|
| Salt A | 20 |
| Salt B | 30 |
| Salt C | 40 |
[Insert graph here]
4. Discussion
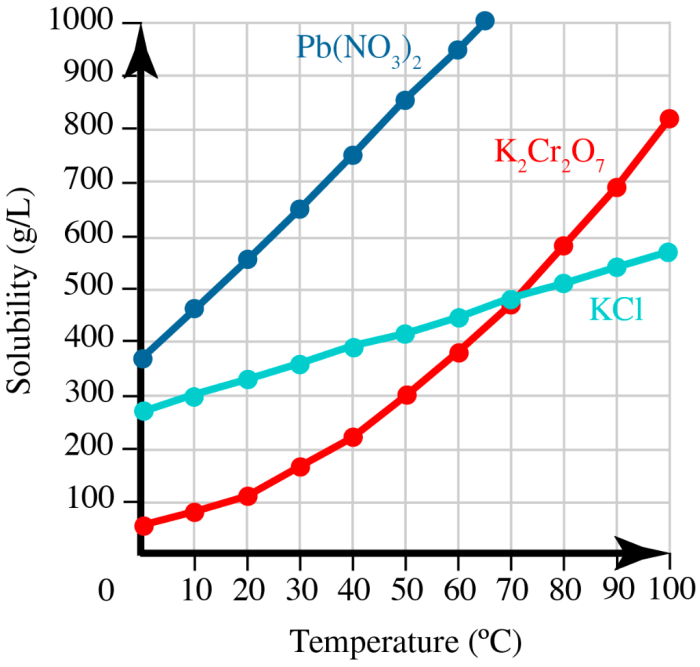
The solubility data reveal that the three salts exhibit varying solubilities in water. Salt C has the highest solubility, followed by Salt B and then Salt A. This variation can be attributed to differences in the salts’ ionic sizes, hydration energies, and interactions with water molecules.
Temperature also plays a role in solubility. Generally, the solubility of salts increases with increasing temperature as the kinetic energy of the solvent molecules increases, leading to more effective solvation of the salt ions.
5. Applications: The Solubilities Of Three Salts In Water
The solubility data has practical applications in various fields:
- Water Treatment:Solubility data helps determine the optimal conditions for water purification and desalination processes.
- Chemical Synthesis:Solubility information is essential for designing crystallization and precipitation reactions used in chemical synthesis.
- Pharmaceutical Formulations:Solubility data guides the development of drug formulations with desired bioavailability and stability.
Questions Often Asked
What factors influence the solubility of salts in water?
Temperature, ion size, and hydration energy are key factors that affect the solubility of salts in water.
How is the solubility of salts measured?
Solubility is typically measured by determining the concentration of a saturated solution, where no more salt can be dissolved at a given temperature.
What are the applications of solubility data?
Solubility data is used in various fields, including water treatment, chemical synthesis, pharmaceutical formulations, and environmental monitoring.

